Why does the heart enlarge? Myosin mutation and hypertrophic cardiomyopathy may be the culprits
The genetic basis of hypertrophic cardiomyopathy was discovered only about a quarter-century ago. Since then, researchers have gathered a wealth of knowledge about the effects of causal mutations. Today, new drugs are available that directly target the molecular basis of the disease. The following text explains how mutations in heart muscle proteins are linked to myocardial hypertrophy.
Hypertrophic Cardiomyopathy and its Manifestations
Hypertrophic cardiomyopathy (HCM) is the most common hereditary heart disease, affecting more than one in every 500 people. It carries significant morbidity in the form of arrhythmias, heart failure, and risk of sudden death. HCM is characterized by left ventricular hypertrophy with a reduced ventricular cavity, which cannot be explained by other pathologies such as aortic stenosis or systemic hypertension.
A Well-Oiled Machine
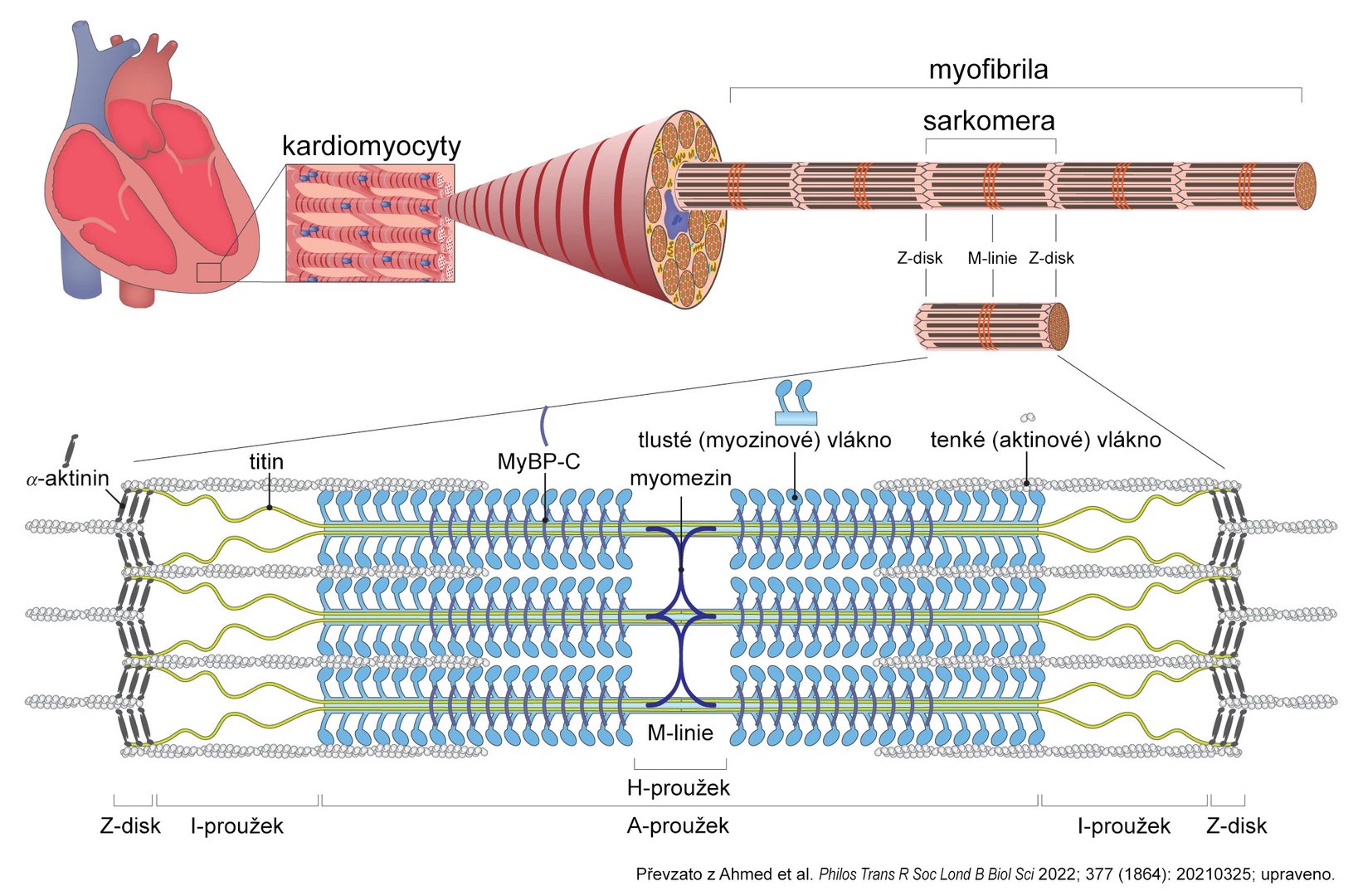
Myocardial contractility is key to heart physiology, determined by both preload and afterload as well as the properties of the cardiomyocyte contractile apparatus. The basic structural unit of this apparatus is the sarcomere, composed of actin and myosin filaments (see figure). Myosin is the main “motor” that utilizes energy from ATP hydrolysis after binding to actin to change its conformation, leading to its movement along the actin filament. This shortens the sarcomere and causes heart muscle contraction.
Other sarcomere proteins, such as myosin-binding protein C (MyBP-C) and titin, modulate the effect and performance of actin-myosin interaction based on physiological stimuli or pharmacological interventions.
Figure: Simplified diagram of sarcomere structure
Why Does the Heart Enlarge?
Many genetic variants of sarcomere proteins are known to cause hypertrophic or dilated cardiomyopathies, suggesting that even slight differences in sarcomere function can lead to significant changes in myocardial morphology and function.
The first discovered mutation related to familial HCM was the R403Q variant in the gene for myosin heavy chain MYH7, described in 1990. Screening candidate genes in families with HCM occurrence and in sporadic cases led to the discovery of mutations in many other genes encoding sarcomere proteins, such as MyBP-C, the troponin complex, and tropomyosin.
Functional characterization of these mutations was a significant scientific challenge due to the difficulty of preserving the enzymatic function of biopsy samples. Additionally, most patients are heterozygous, so their myocardium contains the physiological variant of the protein as well.
From the Laboratory to the Patient's Bedside
Researchers created several animal models, but these provided mixed results. Recombinant human heart myosin was also produced, but these in vitro experiments were inconclusive. Bioinformatics studies recently hypothesized that most causal mutations in the myosin motor domain weaken interactions necessary to maintain myosin in an “off” conformational state, thereby increasing the availability of myosin heads for interaction with actin.
Based on the hypothesis that HCM is rooted in hypercontractility at the sarcomere level, a selective low-molecular-weight myosin activity inhibitor, mavacamten, was developed. The substance was first tested on three different HCM mouse models, where early application prevented hypertrophy development. In older mice, it led to the regression of already developed hypertrophy. This reversible allosteric inhibitor stabilizes myosin in the “off” state. Mavacamten then successfully passed phase I–III clinical trials and, in June 2023, received approval from the European Medicines Agency (EMA) for the treatment of symptomatic hypertrophic obstructive cardiomyopathy in adult patients with heart failure functional class II–III according to the NYHA classification.
(este)
Sources:
1. Kawana M., Spudich J. A., Ruppel K. M. Hypertrophic cardiomyopathy: mutations to mechanisms to therapies. Front Physiol 2022; 13 : 975076, doi: 10.3389/fphys.2022.975076.
2. Ahmed R. E., Tokuyama T., Anzai T. et al. Sarcomere maturation: function acquisition, molecular mechanism, and interplay with other organelles. Philos Trans R Soc Lond B Biol Sci 2022; 377 (1864): 20210325, doi: 10.1098/rstb.2021.0325.
3. SPC Camzyos. Available at: https://ec.europa.eu/health/documents/community-register/2023/20230626159388/anx_159388_cs.pdf
Abbreviated product information can be found here - https://www.bms.com/assets/bms/cz/en/documents/CAMZYOS_ZIP.pdf
3500-CZ-2300031
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
