Off-label use of eye drops containing bromfenac in the treatment of cystoid macular edema in the only seeing eye of a complex patient – case report
The following case report discusses the effect of repeated use of an ophthalmic preparation containing bromfenac in a patient with recurrent cystoid macular edema (CME) in the only seeing eye with advanced glaucoma. The patient has undergone more than 15 eye surgeries or procedures, has been treated for glaucoma since 2005, and has multiple intolerances to eye drops and polymorphic problems, which she attributes to the application of the drops. The dominant personality trait is an anxious approach, which, together with the complicated findings in the only seeing eye, narrows the selection of therapeutic options.
Medical History
The patient, born in 1962, was diagnosed with glaucoma in 2005. From the beginning of the treatment, she reported numerous intolerances to antiglaucoma medications. In 2005 and 2008, selective laser trabeculoplasty was indicated for both eyes, and in 2009 and 2010, filtering surgery with the application of mitomycin was performed sequentially on both eyes with temporary effect. The patient underwent cataract surgery on both eyes, followed by a second trabeculectomy bilaterally, with repeated revisions on the left. Due to CME after cataract surgery, an anti-VEGF antibody was injected intravitreally once in 2020 with good effect. Triamcinolone was repeatedly injected into the left eye for recurrent CME, without effect. Due to decompensation of intraocular pressure (IOP) in the left eye, the patient underwent cyclophotocoagulation three times. The most recent procedure was Nd-YAG capsulotomy of the posterior capsule combined with the release of anterior capsule phimosis in the right eye (March 2023).
Initial Examination and Therapy
The patient came to our clinic on August 8, 2023, for worsening vision in the only seeing right eye.
Initial visual acuity in the right eye (VOR) was 0.5 ccs (worsening from 0.6–0.7 at previous check-ups), no improvement with correction, and visual acuity in the left eye was light perception with certain light projection. Intraocular pressure was borderline compensated – IOP right eye 21–24 mmHg (CCT right eye 598, left eye not reliably measurable) with chronic medication (timolol eye drops 2x daily, dorzolamide eye drops 2x daily, preservative-free artificial tears 3–5x daily, hydrocortisone eye drops 1–2x daily in the right eye, fluconazole eye drops 2x daily in the left eye).
Examination findings:
Right eye: calm conjunctiva, old filtering pillow, intact cornea, calm, moderately deep anterior chamber, calm iris, nasal PAS at III peripheral, free pupil, intraocular lens (IOL) in situ, fenestrated visual field.
Left eye: bulb with chronic hyperemia, old filtering pillow, cornea with chronic greying and old pigmented precipitates on the endothelium, calm anterior chamber, irregular pupil, IOL in situ, fundus not reliably examinable (stationary state).
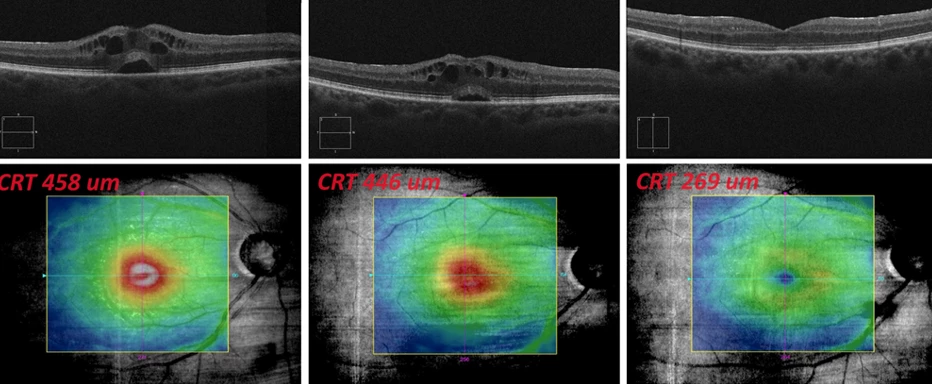
Fundus examination with optical coherence tomography (OCT) of the right eye showed CME with small sensory epithelium detachment under the center, minimal epiretinal membrane paracentrally, central retinal thickness (CRT) 458 μm, and optical coherence tomographic angiography (OCTA) without signs of choroidal neovascularization (CNV).
The diagnosis was new CME attack, considering a possible late reaction to YAG capsulotomy. Therapy with a dietary supplement containing horse chestnut, silver birch, zinc, and lutein was initiated, along with nepafenac eye drops 3 mg/ml once daily, and chronic medication was unchanged.
Follow-up Examinations Chronologically
August 24, 2023
Subjective: worsening vision, does not tolerate nepafenac, feeling of sticky eye, vision further blurring.
Objective: VOR worsened to 0.36 ccs, IOP right eye 17 mmHg, OCT and biomicroscopically CME identical, slight CRT decrease to 446 μm, recommended fluorescein angiography (FAG) – patient refused due to fear of possible side effects.
Recommended: change therapy from nepafenac to bromfenac eye drops twice daily, maintain other medications.
September 25, 2023
Subjective: reports using bromfenac for 14 days exactly as per the leaflet, then stopped, now satisfied.
Objective: VOR 0.7-1 ccs, IOP right eye 18 mmHg, biomicroscopically and on OCT CME in remission, CRT 269 μm.
Recommended: continue chronic medication and dietary supplement with horse chestnut, silver birch, zinc, and lutein 1 tablet daily.
Figure 1 OCT findings progression in the macula of the right eye: August – September 2023
December 11, 2023
Subjective: recurring worsening vision in the right eye.
Objective: VOR 0.45 ccs, IOP right eye 16 mmHg, OCT shows recurrence of CME, CRT 567 μm.
Recommended: bromfenac eye drops twice daily, return dietary supplement with horse chestnut, silver birch, zinc, and lutein to 1-0-1 dosage.
December 28, 2023
Subjective: no change, sees patterns before eyes, used bromfenac precisely for 14 days.
Objective: VOR 0.36 ccs, IOP right eye 15 mmHg, partial regression of CME on OCT, CRT 433 μm.
Recommended: two-week break, then again bromfenac eye drops twice daily for 14 days, follow-up after completion.
January 26, 2024
Subjective: perceives some improvement but still not satisfactory, requests to discontinue corticosteroid (CS) and change antiglaucoma therapy, no longer wants to use bromfenac.
Objective: VOR 0.6-2 ccs, IOP right eye 19 mmHg, CME reduction, residual edema, CRT 341 μm.
Recommended: after discussion with the patient, nepafenac eye drops 3 mg/ml once daily, change antiglaucoma therapy to dorzolamide/timolol twice daily.
Figure 2 OCT findings progression in the macula of the right eye: December 2023 – January 2024

February 14, 2024
Reports by phone that nepafenac bothers her more than bromfenac, requests another course of bromfenac.
February 23, 2024
Subjective: satisfactory vision but again irritated eyes.
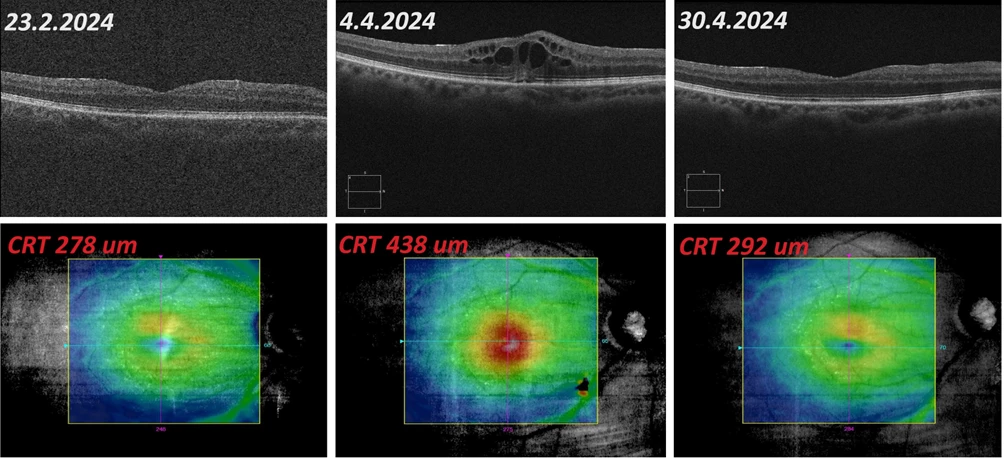
Objective: VOR 0.6-2 ccs, IOP right eye 16 mmHg, biomicroscopically and on OCT CME resolved, CRT 278 μm.
Recommended: continue chronic medication, increase lubricants, and dietary supplement with horse chestnut, silver birch, zinc, and lutein one tablet daily.
April 4, 2024
Subjective: worsening vision, overall medication change from bupropion to venlafaxine, not tolerating well, feels very unwell.
Objective: VOR 0.1 ccs, IOP right eye 16 mmHg, biomicroscopically and on OCT recurrence of CME, CRT 438 μm.
Recommended: bromfenac eye drops twice daily, advised to leave on longer, patient refuses.
April 30, 2024
Subjective: better vision, reports dorzolamide/timolol not well tolerated, feels eyelids tingling, requests change in therapy, used bromfenac for 14 days.
Objective: VOR 0.6 ccs, IOP right eye 17 mmHg, CME reduction, only residual edema in the lower macula on OCT, CRT 292 μm.
Recommended: change antiglaucoma therapy to preservative-free dorzolamide/timolol, next follow-up planned in one month.
Figure 3 OCT findings progression in the macula of the right eye: February – April 2024
Discussion and Conclusion
In August 2023, the patient experienced a new episode of CME, which was initially diagnosed after cataract surgery in the right eye in 2020 and successfully treated with intravitreal anti-VEGF preparation. Possible etiology for repeated CME onset includes a late reaction to the performed YAG capsulotomy of the anterior and posterior capsules (March 2023); however, precise causal examination was not possible due to the patient's refusal of FAG.
Treating CME in this complex patient posed several challenges – primarily, it is the only seeing eye with advanced glaucoma and poor tolerance to conservative therapy. Intravitreal corticosteroid administration (or depot CS) carries a high risk of intraocular pressure decompensation and endophthalmitis. Off-label intravitreal anti-VEGF application was discussed with the patient, but she refused due to fear of surgery. Another major complication is multiple intolerances and low treatment adherence.
Eventually, off-label use of bromfenac eye drops twice daily in combination with systemic antiedematous therapy proved effective and safe. Although adherence to treatment was not ideal, the patient tolerated the drops relatively well, with repeated symptom relief. Despite recurring CME, the patient refuses dose adjustments to prevent recurrence. During bromfenac use, no intraocular pressure elevation in the right eye was observed, which is vital considering the advanced glaucomatous changes, and no objective side effects were noted.
Ondřej Hrbáček, M.D.
Ophthalmology Department, Litomyšl Hospital
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
