Bimekizumab in 3rd Line Biological Treatment After Risankizumab Failure – Case Study
The possibilities of biological therapy for severe forms of psoriasis are very broad today. Achieving remission or reducing disease activity by 90-100% from the baseline state (PASI 90-100) is highly probable, especially when using biological drugs from the IL-17 or IL-23 inhibitor groups. However, individually, patients may exhibit primary or secondary resistance even when using these preparations, necessitating a change in biological treatment. The following case study presents the case of a young man who achieved complete disease remission with bimekizumab after the previous failure of ixekizumab and risankizumab.

Case Description
A 37-year-old patient with a history of psoriasis since the age of 25 was initially treated with standard external methods in a regional dermatology clinic due to the milder form of the disease. Apart from being overweight (weighing 100–110 kg at 186 cm height), he had no other comorbidities at the time of the psoriasis diagnosis. In 2015, he was newly diagnosed with and treated for arterial hypertension.
The manifestations of psoriasis progressively worsened, and external therapy became ineffective. Repeated courses of whole-body UVB 311 nm phototherapy and methotrexate therapy failed to induce disease remission, leading to the initiation of biological therapy with an IL-17 inhibitor, ixekizumab, in August 2020. This therapy induced disease remission, achieving PASI 90 by week 8 and complete remission (PASI 100) by week 12. However, from week 36 of ixekizumab treatment, mild signs of increased disease activity appeared in the form of smaller inflammatory lesions on the lower legs. Disease activity escalated, and by week 92, when PASI 50 was no longer achieved, ixekizumab administration had to be discontinued.
In June 2022, treatment with the IL-23 inhibitor risankizumab was initiated. The onset of effect was very slow, with partial remission (PASI 75) achieved by week 16, which lasted until week 28. Subsequently, the effect of risankizumab waned, and the treatment was halted by week 40 due to insufficient efficacy.
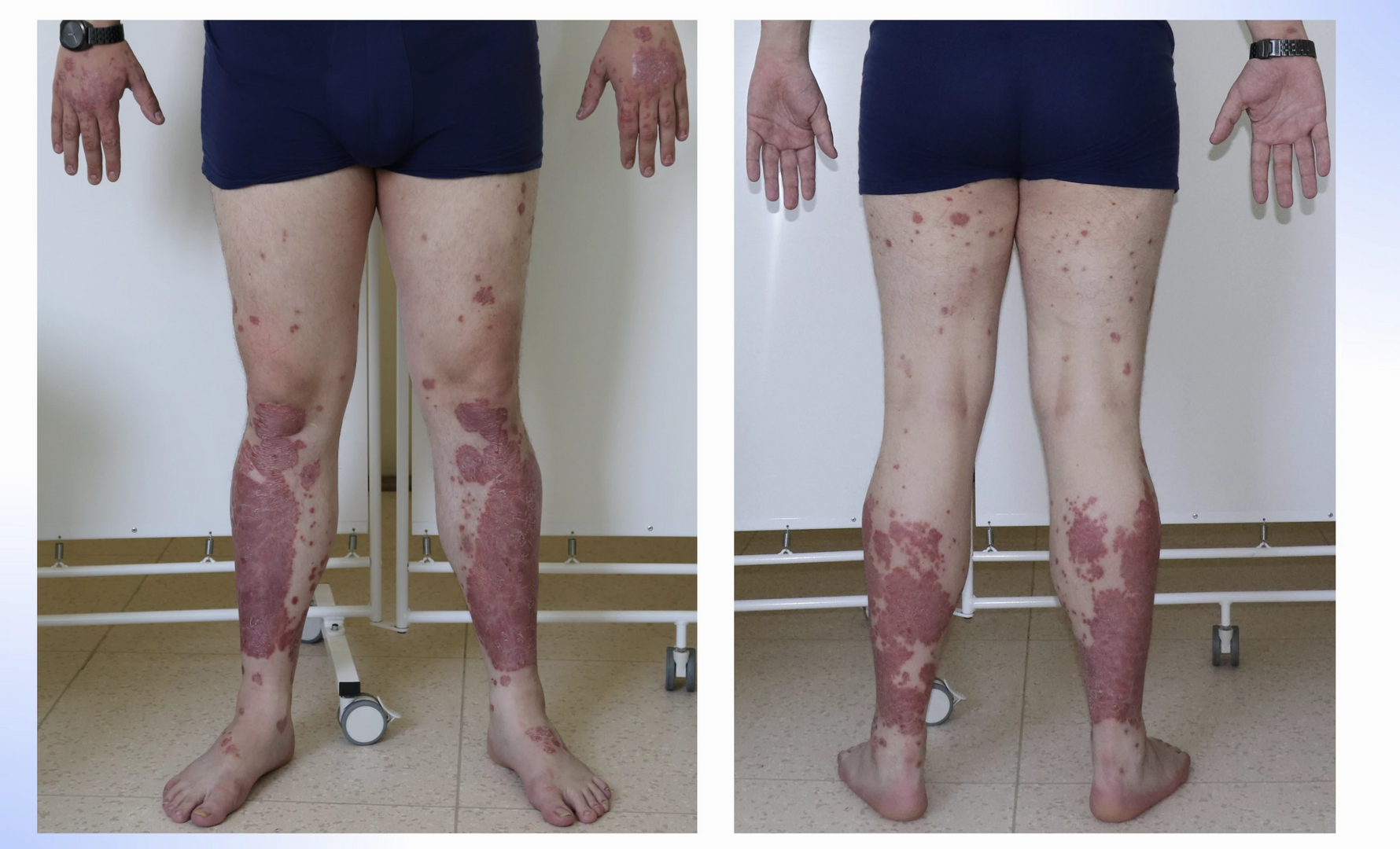
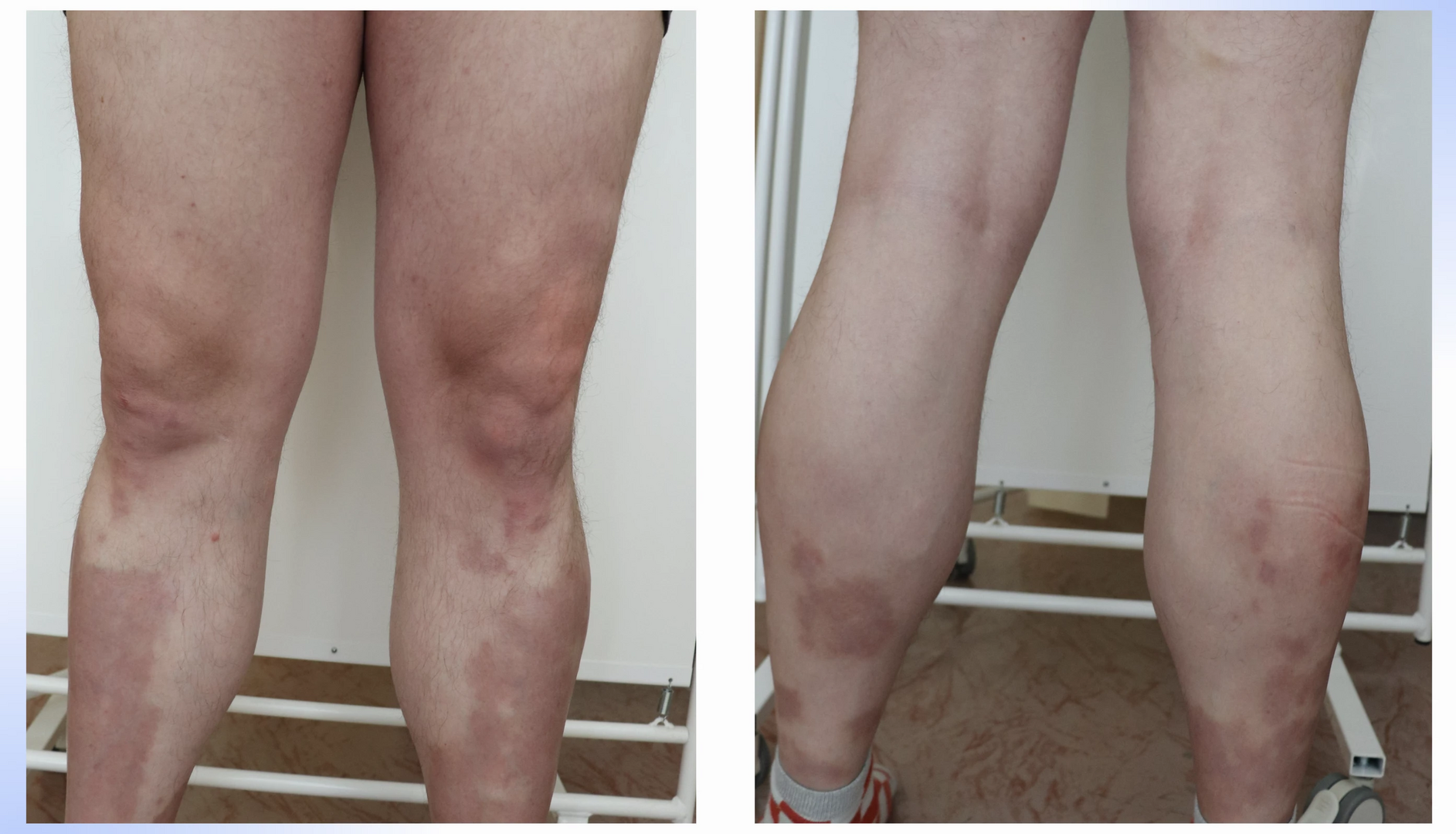
Given the failure of two biologics generally exhibiting high treatment success, another therapeutic approach was considered. Bimekizumab, another IL-17 inhibitor, which, unlike ixekizumab, also blocks IL-17F activity, was chosen. This strategy proved successful. A notable characteristic of the entire IL-17 inhibitor group is the rapid onset of effect, particularly marked with bimekizumab. At the start of this treatment, the baseline PASI was 10.8, with predominant manifestations on the lower extremities (see Fig. 1, 2), but by week 4, near-complete disease remission with residual post-inflammatory changes (PASI 0.8) was achieved (see Fig. 3, 4). Currently, the patient is in the 48th week of bimekizumab therapy and complete disease remission persists in the form of PASI 100 (absolute PASI 0), with all negative impacts on quality of life measured by the Dermatologic Life Quality Index (DLQI) completely eliminated.
Conclusion
The high efficacy of bimekizumab in biologically naïve patients has been demonstrated in many registration studies and is confirmed by initial observations from real-world practice. Its unique mechanism of action is proving to be very advantageous when choosing therapeutic strategies in higher lines of biological treatment. The presented case suggests that dual inhibition of IL-17A and IL-17F by bimekizumab has high potential not only for intra-class transfers within the IL-17 inhibitor group but also for patients with primary or secondary resistance to IL-23 inhibitor preparations.
MUDr. Martin Tichý, Ph.D.
Clinic of Skin and Venereal Diseases, Faculty of Medicine UP and University Hospital Olomouc
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
