Treatment of Low-Risk MDS According to NCCN Recommendations Version 1.2023
The latest recommendations from the American National Comprehensive Cancer Network (NCCN) for the diagnosis and treatment of myelodysplastic syndrome (MDS) include several updates incorporated into the graphical algorithms. Most patients with MDS have low-risk (LR) disease. We provide a summary of the current guidelines NCCN for the therapy of this group of patients, which differ depending on the presence of symptomatic anemia or clinically relevant thrombocytopenia or neutropenia.
Low-Risk MDS
Low-Risk MDS (MDS-LR) is defined based on the IPSS-R (Revised International Prognostic Scoring System) score value in the very low, low, and intermediate ranges, i.e., ≤ 3.5. Treatment is recommended for patients with clinically significant cytopenia. Supportive care is an integral part of therapy.
Treatment of Patients with MDS-LR and Clinically Relevant Thrombocytopenia or Neutropenia
In the case of clinically relevant thrombocytopenia or neutropenia, azacitidine is preferred according to the recommendations, or the patient can be enrolled in a clinical trial. Other recommended modalities are intravenous decitabine or the combination of oral decitabine + cedazuridine. Immunosuppressive therapy with the possible addition of eltrombopag can be beneficial under certain circumstances. If the disease progresses or there is no response to treatment, hypomethylating agents, clinical trial participation, and allogeneic hematopoietic cell transplantation in selected patients can be considered.
Treatment of Patients with MDS-LR and Symptomatic Anemia
The recommended approach for patients with symptomatic anemia is shown in the following diagram.
Fig. NCCN Recommendations (version 1. 2023) for the treatment of Low-Risk MDS with symptomatic anemia (all recommendations are class 2A)
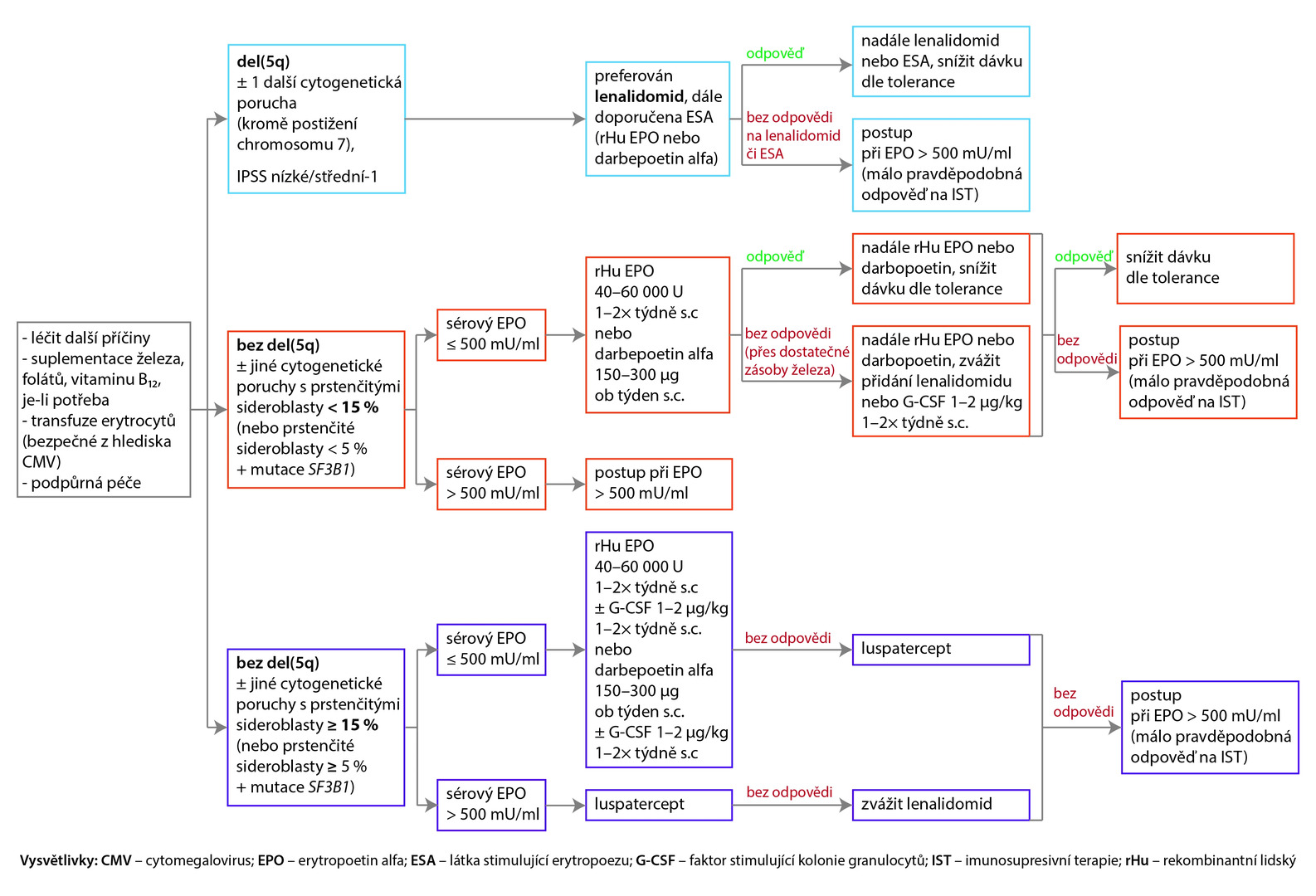
Approach with Serum Erythropoietin Levels > 500 mU/ml
If serum erythropoietin levels exceed 500 mU/ml and the probability of response to immunosuppressive therapy is sufficiently high, the preferred recommendation is antithymocyte globulin (ATG) + cyclosporine A + eltrombopag, or ATG + cyclosporine A can be administered; another recommended option is ATG + eltrombopag, or under certain circumstances, ATG alone can be used (recommendation class 3). For patients without response or with intolerance to this treatment, it is recommended to proceed as with a low probability of response to immunotherapy.
In the case of serum erythropoietin levels > 500 mU/ml and low probability of response to immunosuppression, azacitidine is the preferred choice, or enrolling in a clinical trial is suitable. Other options are decitabine or oral decitabine and cedazuridine. Lenalidomide can be considered under certain circumstances. If there is no treatment response after 6 cycles of azacitidine or 4 cycles of decitabine or oral decitabine and cedazuridine, or if the patient is intolerant to this treatment, enrolling in a clinical study is recommended, or allogeneic hematopoietic stem cell transplantation can be considered for selected patients.
(zza)
Source: Myelodysplastic Syndromes. NCCN Clinical Practice Guidelines in Oncology. Version 1.2023. Available at: www.nccn.org/guidelines/guidelines-detail?category=1&id=1446
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
