Stabilization of Transthyretin and Long-term Survival of Patients with Cardiac Amyloidosis
Tafamidis is used in the therapy of transthyretin amyloid cardiomyopathy (ATTR-CM), which has proven its benefit in the ATTR-ACT clinical study. The results of its open-label extension study show that early diagnosis and prompt initiation of therapy are crucial.
Transthyretin Amyloidosis – How Does Tafamidis Help?
In ATTR-CM, native tetramers of the transport protein transthyretin (TTR) break down into monomers, which then aggregate into amyloid fibrils that deposit among other places in the heart muscle tissue. The hereditary variant of the disease (ATTRv) is the result of mutations in the TTR gene. ATTRwt, formerly known as senile cardiac amyloidosis, is caused by the natural destabilization of TTR tetramers associated with aging. The median survival from diagnosis is approximately 2.5 years for ATTRv and about 3.6 years for ATTRwt.
The selective stabilizer tafamidis prevents the breakdown of TTR tetramers and thus the formation of amyloid. In the multicenter double-blind placebo-controlled ATTR-ACT study, tafamidis reduced mortality and the number of hospitalizations for cardiovascular causes over 30 months of use.1 Patients from the ATTR-ACT study could participate in the open-label extension, where all participants take tafamidis for up to 60 months.2
Methodology and Course of the Study
Upon entering the open-label extension, patients from the experimental arm continued taking tafamidis meglumine salt at a dose of 20 mg (n = 88; these participants are not included in the presented analysis) or 80 mg (n = 110). Patients from the placebo arm were randomized 2:1 to receive tafamidis meglumine salt at a dose of 20 mg (n = 28) or 80 mg (n = 54). In July 2018, the protocol was amended, and all participants were switched to tafamidis free acid at a dose of 61 mg (bioequivalent to 80 mg of the meglumine salt).
The primary objective of the interim analysis was all-cause mortality, which included heart transplant or mechanical heart support device implantation. Both events from the primary study and its extension were included.
Results
The median follow-up time was 58.5 months in the continuous tafamidis treatment arm (n = 176) and 57.1 months in the group switched from placebo (n = 177). All-cause mortality was 44.9% for continuously tafamidis-treated patients and 62.7% in the placebo-sequence tafamidis arm (hazard ratio [HR] 0.59; 95% confidence interval 0.44–0.79; p < 0.001) (see fig.).
Fig. Long-term survival of ATTR-ACT study participants and its open-label extension
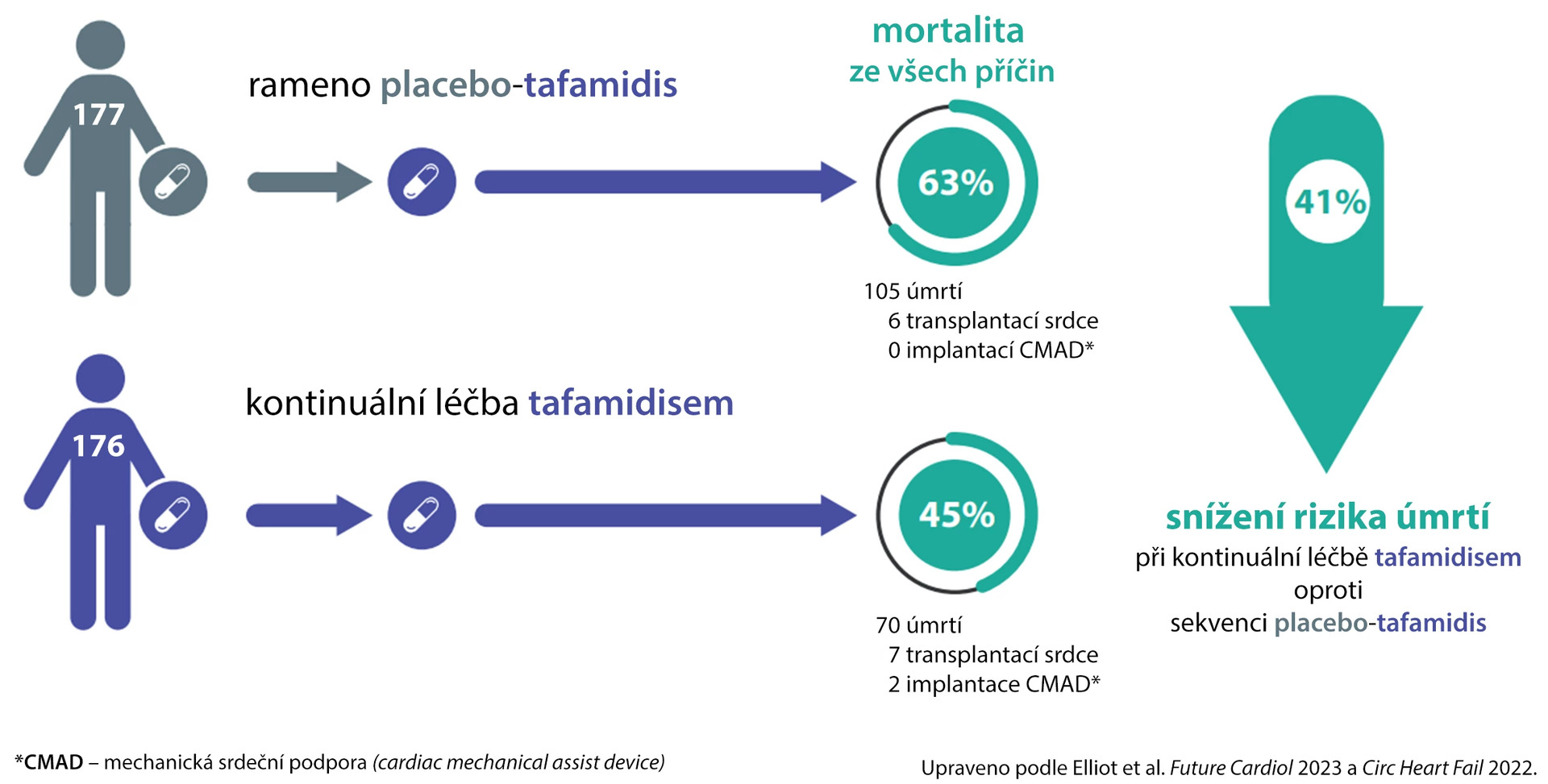
Mortality was lower with continuous tafamidis treatment across all subgroups:
- in patients with ATTRv: HR 0.57 (95% CI 0.33–0.99; p = 0.05)
- ATTRwt: HR 0.61 (95% CI 0.43–0.87; p = 0.006)
- NYHA class I and II heart failure: HR 0.56 (95% CI 0.38–0.82; p = 0.003)
- NYHA III heart failure: HR 0.65 (95% CI 0.41–1.01; p = 0.06)
Conclusion
The open-label extension of the ATTR-ACT study showed that the survival of patients who started tafamidis therapy in the original study (2.5 years earlier) was significantly longer compared to those who received placebo in the original study. These results highlight the critical importance of early diagnosis and initiation of therapy.
(este)
Sources:
1. Maurer M. S., Schwartz J. H., Gundapaneni B. et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018; 379 (11): 1007–1016, doi: 10.1056/NEJMoa1805689.
2. Elliott P., Drachman B. M., Gottlieb S. S. et al. Long-term survival with tafamidis in patients with transthyretin amyloid cardiomyopathy. Circ Heart Fail 2022; 15 (1): e008193, doi: 10.1161/CIRCHEARTFAILURE.120.008193.
3. Elliott P., Drachman B. M., Gottlieb S. S. et al. Long-term survival in people with transthyretin amyloid cardiomyopathy who took tafamidis: a plain language summary. Future Cardiol 2023 Jan; 19 (1): 7–17, doi: 10.2217/fca-2022-0096.
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
