Role of Transthyretin in the Development of Amyloidosis
Transthyretin (TTR), one of the transport proteins, tends to form extracellular deposits that accumulate in key organs, such as the heart and nerves. The formation of TTR deposits leads to the development of systemic amyloidosis, a disease with highly heterogeneous clinical manifestations. Warning signs may include carpal tunnel syndrome, among others. What is the physiological role of TTR in the body and what role does its structure play in the development of amyloidosis?
Physiological Function of TTR
TTR was first discovered in cerebrospinal fluid (CSF) and serum. Its function is to transport the hormone thyroxine (T4). In plasma, TTR mediates the transfer of 15% of T4, while in CSF this number is as high as 80%. TTR also specifically binds to retinol-binding protein (RBP). It is believed that the binding of TTR to RBP prevents the loss of RBP during glomerular filtration in the kidneys.
TTR is synthesized in various tissues, such as the liver, choroid plexus of the brain, alpha cells of the pancreas, and retinal pigment epithelial cells. The concentration of TTR varies in different tissues, constituting 25% of total protein in the CSF. The plasma concentration of TTR is higher in adulthood than in childhood and higher in men than in women. TTR is used as an indicator of sufficient energy and protein levels.
Structure of TTR
The gene for the TTR protein encodes 147 amino acids, of which the first 20 contain a signal sequence that is cleaved before extracellular transport. Mature TTR contains 127 amino acids and forms a homotetramer with a central channel. Although there are 2 binding sites for T4 on the TTR tetramer, only one molecule of T4 binds due to negative cooperativity. A similar situation occurs with RBP, where 2 molecules of RBP with bound retinol can simultaneously bind to the TTR tetramer.
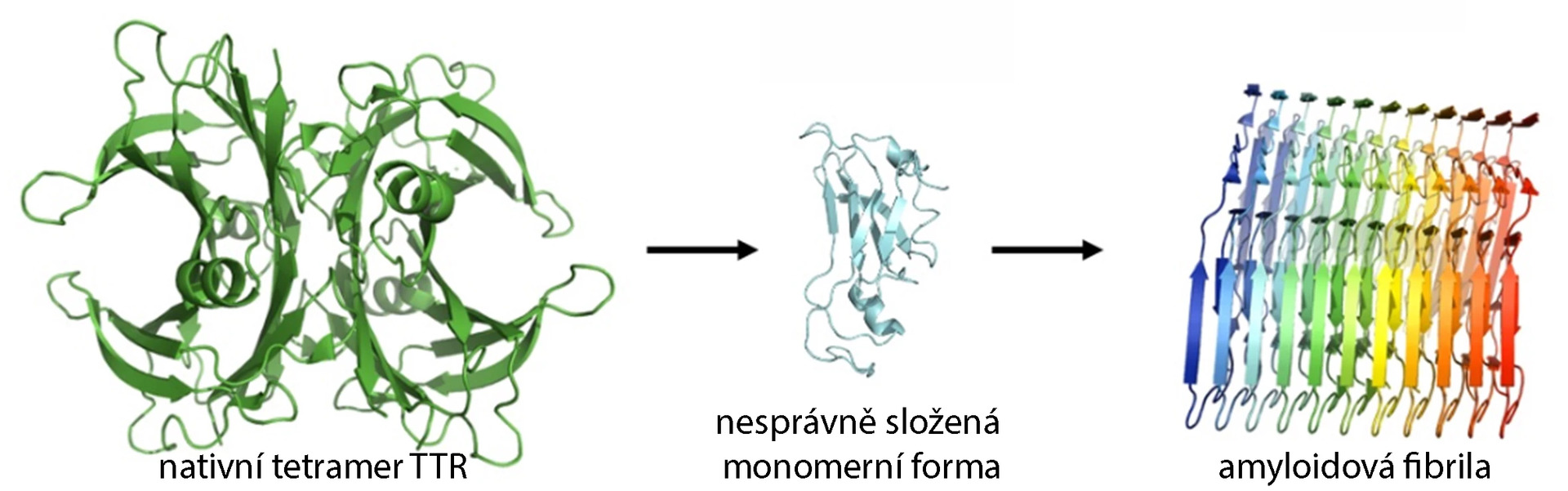
The primary structure of TTR is evolutionarily highly conserved. Mutations in the TTR gene in the vast majority of cases cause the destabilization of the TTR tetramer, which is considered a key step in the formation of amyloid and the development of amyloidosis. The tetramer itself does not form deposits; however, its dissociation into monomers can lead to amyloid formation and the development of amyloidosis.
Fig. Dissociation of the TTR tetramer and formation of amyloid fibrils (adapted from Saponaro et al.)
Systemic Amyloidosis and Its Manifestations
Two types of systemic amyloidosis are described − hereditary familial ATTR amyloidosis caused by a mutation in the TTR gene and the so-called ATTR-wt amyloidosis, which occurs in individuals without pathological TTR mutations at an older age.
In patients with hereditary systemic amyloidosis, sensory-motor polyneuropathies, autonomous dysfunctions, heart failure, gastrointestinal tract disorders, and other symptoms are found, which in the majority of cases, lead to death within 10 years from the onset of the disease. The disease usually manifests in individuals over the age of 40 (depending on the type of mutation), with a median survival from diagnosis for untreated patients of about 2 years.
Symptoms of senile systemic amyloidosis include cardiomyopathy, carpal tunnel syndrome, spinal canal stenosis, and neuropathy in the elderly. The disease typically manifests after the age of 65 and is slightly more common in men. The median survival from the time of diagnosis for untreated patients is approximately 43 months.
Cardiomyopathy caused by transthyretin amyloidosis (ATTR-CM) represents a serious disease that occurs most often in its acquired form in the Czech Republic. Early diagnosis and subsequent therapy are key to improving the prognosis of affected patients.
(eko)
Sources:
1. Ueda M. Transthyretin: its function and amyloid formation. Neurochem Int 2022; 155: 105313, doi: 10.1016/j.neuint.2022.105313.
2. Saponaro F., Kim J. H., Chiellini G. Transthyretin stabilization: an emerging strategy for the treatment of Alzheimer's disease? Int J Mol Sci 2020; 21 (22): 8672, doi: 10.3390/ijms21228672.
3. Cardiac amyloidosis program. UC Davis Health, 2022. Available at: https://health.ucdavis.edu/internalmedicine/cardio/cardiac-amyloidosis-program/more-info.html#Familial
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
