Use of Brodalumab in the Treatment of Severe Plaque Psoriasis − Case Report
The case report by MUDr. Jan Šternberský, Ph.D., from the Clinic of Skin and Venereal Diseases, UP Faculty of Medicine and University Hospital Olomouc, demonstrates the excellent effect of brodalumab in a patient with severe plaque psoriasis resistant to previous treatment modalities.
Personal History
The patient is a 55-year-old man diagnosed with severe plaque psoriasis in 2007. There is no family history of psoriasis; the trigger for this patient was likely significant stress. The man is also being treated for hypertension (perindopril 5 mg) and since 2016 for type 2 diabetes mellitus (dulaglutide s.c. once a week). He takes esomeprazole 40 mg for upper dyspeptic syndrome. He denies any allergies and does not smoke. He works as a car mechanic.
Course of Psoriasis Therapy
The skin disease initially manifested in predilection areas with gradual generalization, and was first treated with topical applications and then whole-body phototherapy. Topical treatment had no effect, and phototherapy was discontinued after a month due to skin irritation and disease progression. In 2013, therapy with acitretin was initiated but was discontinued after 3 weeks due to disease progression and significant side effects (vomiting and headaches).
In subsequent years, the patient participated in several clinical trials with biological treatments (brodalumab, ixekizumab, guselkumab). All therapies had a good effect, but during the last evaluation with guselkumab, mild psoriasis symptoms persisted, and after the treatment ended, there was a relapse and significant progression within 2 months. Therefore, in January 2019, the patient was hospitalized, and systemic therapy with methotrexate was initiated (initially 10 mg weekly, later increased to 20 mg weekly due to insufficient effect). Initially, there was partial efficacy, but eventually, progression was observed again, and methotrexate was discontinued after 5 months.
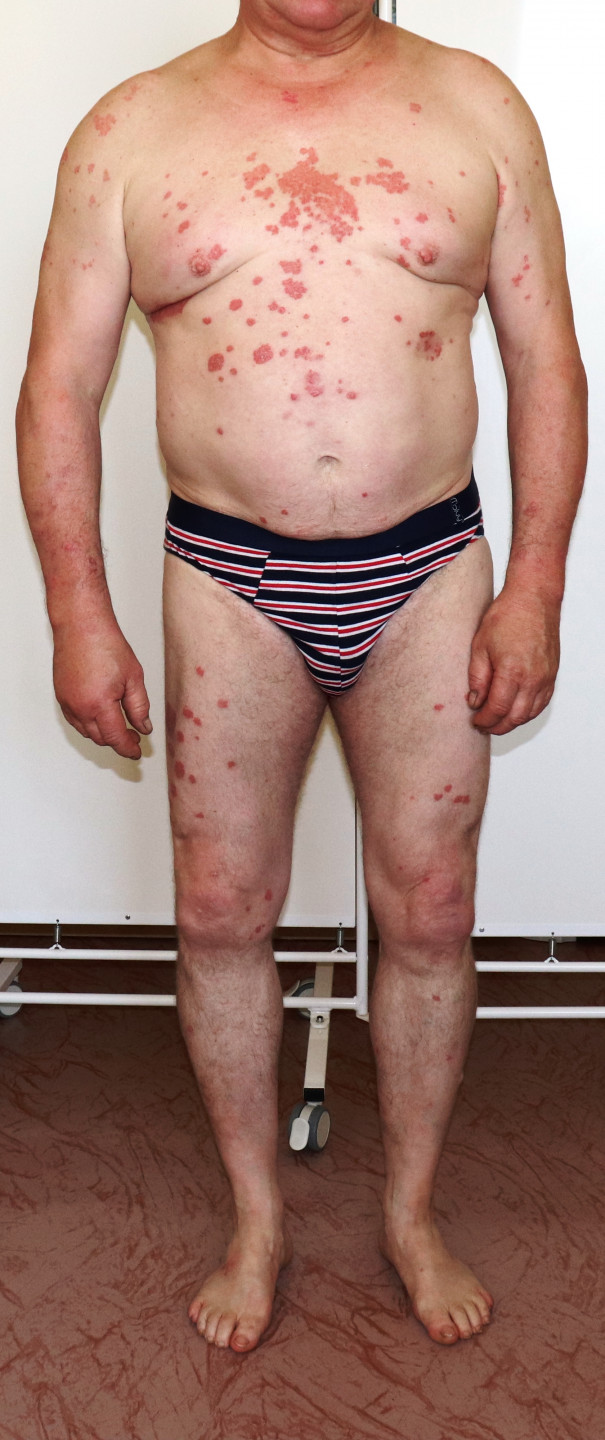
Condition before brodalumab therapy
Change of Therapy and Its Evaluation
In a patient with psoriatic lesions on the upper and lower limbs, trunk, scalp, and nail beds, a PASI score of 17.2 was found. The trunk involvement extent reached up to 69%. Given previous experiences with various treatment methods, brodalumab therapy was reinitiated, as it had the best effect in the past.
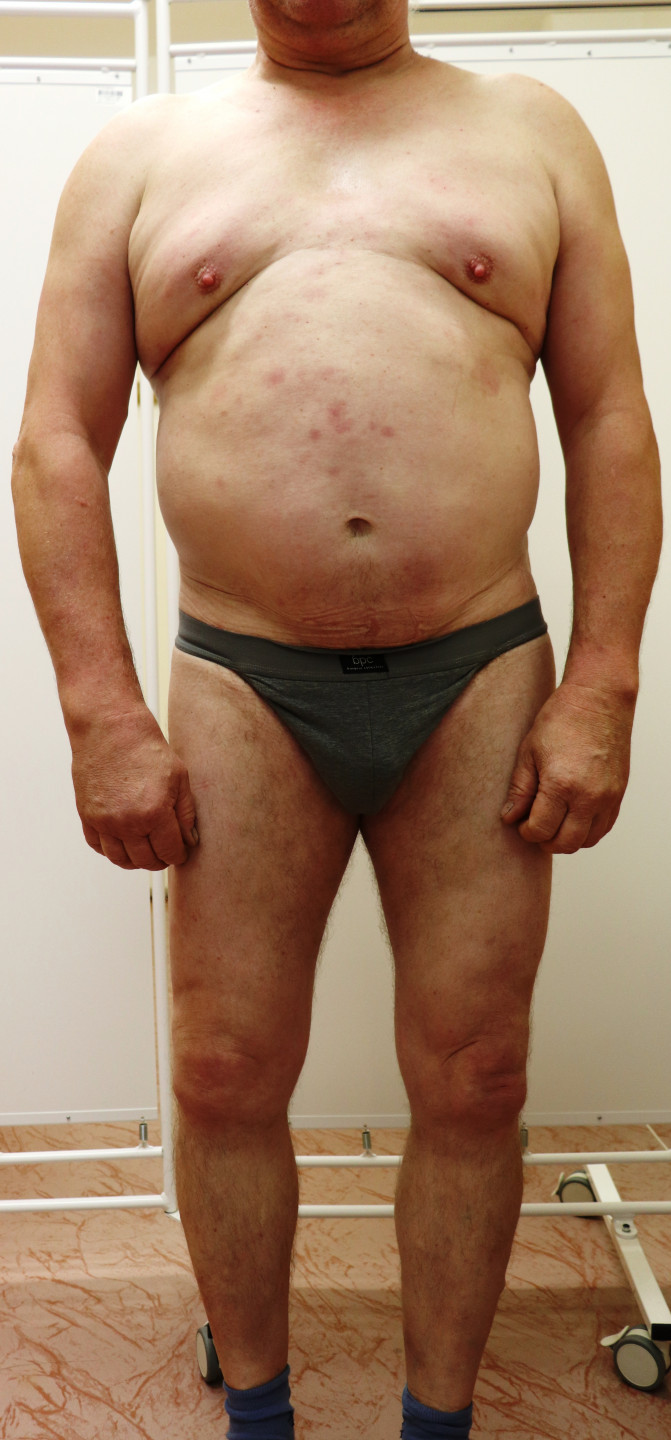
The patient noticed the effect of therapy after the second dose of brodalumab; subcutaneous injection administration was managed without problems, and no side effects were observed. After 4 weeks of treatment, all psoriatic lesions reduced, desquamation and smoothing occurred, and the PASI score dropped to 2.1, thus decreasing by more than 87%. After 12 weeks, further improvement was observed with a 94% reduction in psoriatic inflammation. Only mild lesions on the trunk and lower limbs persisted, with a PASI score of 1.0. At the 48-week mark from the start of brodalumab therapy, complete remission of the disease was achieved.
Significant improvement occurring
Summary and Conclusion
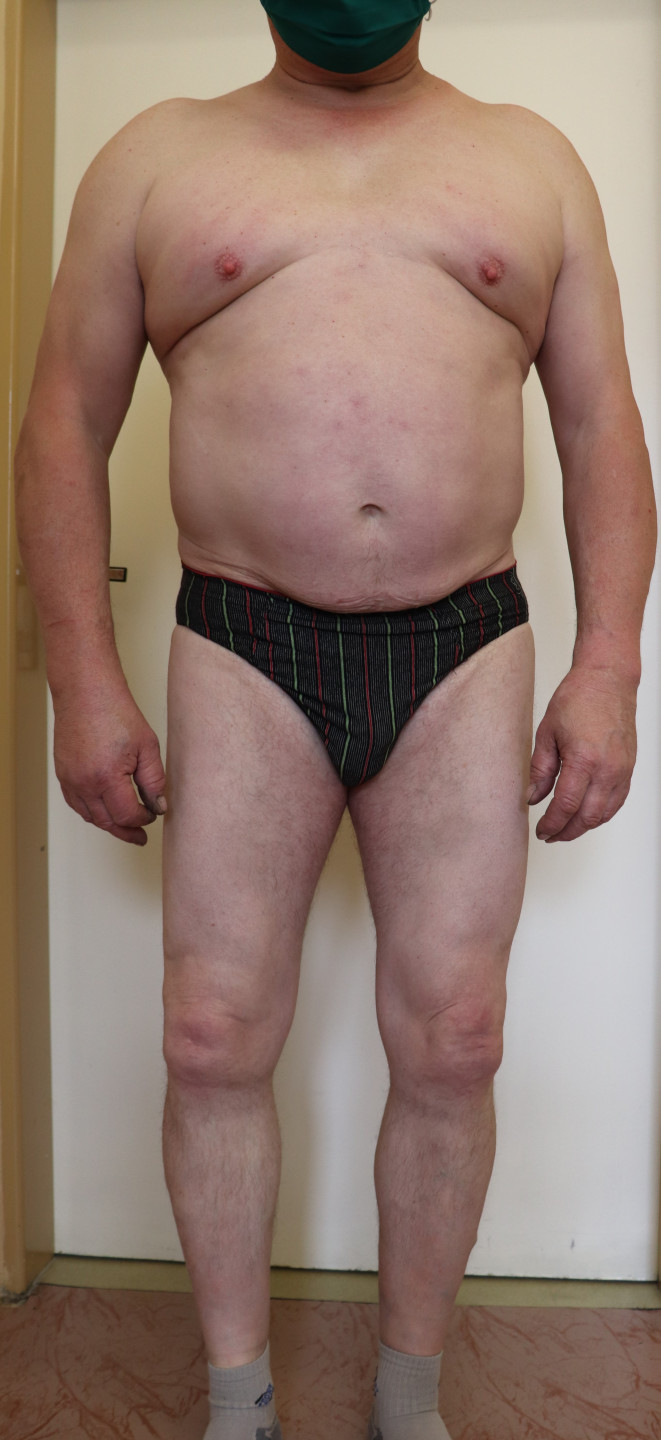
The patient, for whom the treatment of severe plaque psoriasis was associated with failures with both topical and systemic therapies, achieved complete remission thanks to brodalumab. The effectiveness, tolerability, and compliance are rated as excellent by both the patient and the treating physician. After one year of therapy, the man is completely free of psoriasis symptoms, the treatment effect persists, and there is overall satisfaction and absence of any side effects.
The skin still remains completely free of psoriasis symptoms
(pak)
Source: Šternberský J. Case report of a patient with resistant severe plaque psoriasis. Clinic of Skin and Venereal Diseases, UP Faculty of Medicine and University Hospital Olomouc, November 2020.
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
Labels
Dermatology & STDs Paediatric dermatology & STDs- How Compassion Fatigue Threatens Doctors and Other Healthcare Workers
- A device the size of a matchbox will help treat obstructive sleep apnea
- Good, Bad, or Ugly? What's the Deal with Eggs?
- Non-Invasive Analysis of Gut Microbiome Biomarkers Enhances Diagnosis of IBD
- New Technology Enables Early Monitoring and Therapy for tPA-Resistant Blood Clots
Recommended for you
- The Wound Healing Process Step by Step and What Can Complicate It
- Pain Relief and Functional Score Improvement in Patients with Low Rheumatoid Arthritis Activity: Baricitinib vs. Adalimumab
- Another Positive Effect of Diosmin − Influence on Oxidative Stress
- Efficacy and Speed of Pain Relief in Rheumatoid Arthritis When Adding Baricitinib to Methotrexate
- How to Choose the Ideal Venotonic or Vasoprotective – Which Properties Are Key?
- Diosmin – Still an Important Modality in the Treatment of Venous Insufficiency
