Brodalumab – 5 Years of Data on Efficacy, Safety, and Quality of Life Improvement in Plaque Psoriasis
Chronic inflammatory diseases, including psoriasis, require therapeutic approaches that maintain efficacy and tolerability even during long-term treatment. One such modern modality is brodalumab, a fully human monoclonal antibody against the A subunit of the interleukin (IL) 17 receptor. A study published last year evaluated its long-term efficacy and safety in patients with moderate to severe plaque psoriasis and also focused on improving their quality of life.
Psoriasis Brings Many Limitations
Although psoriasis primarily manifests in the form of dermatological lesions, the disease has much broader implications for patients. It negatively impacts health-related quality of life and emotional well-being, increases the risk of comorbidities and mortality, raises the likelihood of suicidal thoughts, creates social stigma, and limits patients in employment and productivity.
In pivotal studies involving patients with moderate to severe plaque psoriasis, brodalumab improved the condition more effectively in terms of skin lesions compared to ustekinumab. Additionally, brodalumab was shown to improve health-related quality of life and alleviate depression and anxiety. The aim of the presented study was to assess the long-term efficacy and safety profile of brodalumab in patients with moderate to severe plaque psoriasis.
Study Methodology
Patients who completed an initial 12-week double-blind, placebo-controlled phase II clinical trial without serious adverse treatment-related events were enrolled in an open-long extension study. In the follow-up long-term study, they received 210 mg of brodalumab subcutaneously every 2 weeks (Q2W). Efficacy was assessed using the static physician's global assessment (sPGA) score and the Psoriasis Area and Severity Index (PASI). The overall report also included quality of life evaluated by the Dermatology Life Quality Index (DLQI) and the safety of brodalumab administration.
Course and Results
A total of 181 patients enrolled in the study, with a median brodalumab administration duration of 264 weeks. As this was a long-term extension of a phase II dose-ranging study, a protocol change allowed patients with a body weight of ≤ 100 kg (65.2% of participants) to reduce the administered dose to 140 mg Q2W. However, since dose reduction was associated with decreased efficacy in 30 patients, another protocol change allowed these patients to increase the dose back to 210 mg Q2W. This step was followed by a return to pre-dose reduction efficacy levels.
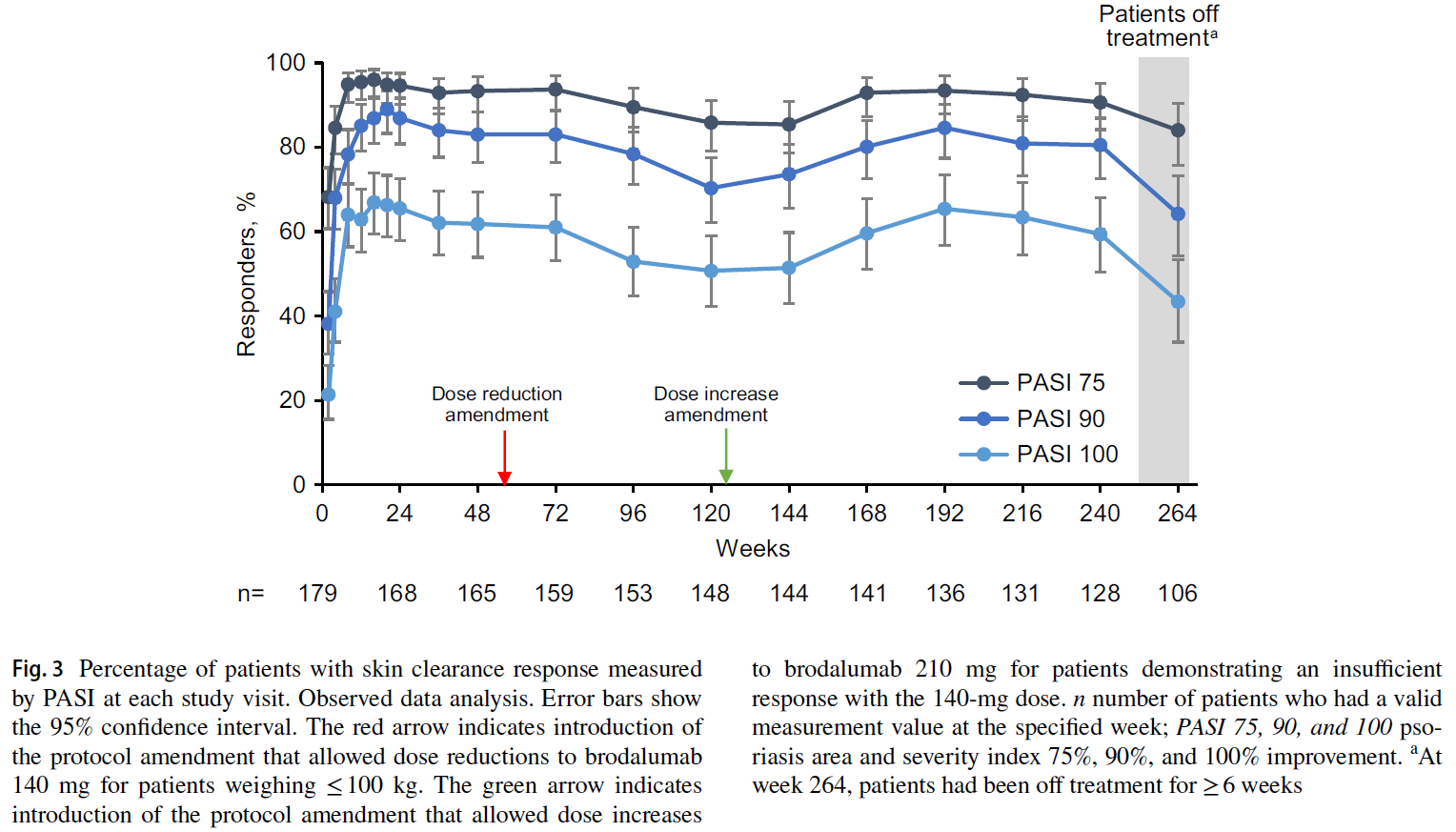
Treatment with brodalumab led to a rapid improvement in sPGA, PASI, and DLQI parameters, which persisted up to week 264. Achieving PASI 90 to < 100 or PASI 100 in weeks 12, 240, and 264 was associated with a higher likelihood of DLQI 0 or 1 compared to patients who achieved PASI 75 to < 90.
The most frequently observed adverse events were nasopharyngitis (29% of patients), respiratory infections (24%), and arthralgia (20%). Over 5 years, there was 1 case of suicidal ideation, no suicides, and no new safety profile insights were observed.
Conclusion
During 5 years of therapy, brodalumab sustainably improved skin lesions and patients' quality of life and showed an acceptable safety profile comparable to short-term treatment.
(mir)
Source: Lebwohl M. G., Blauvelt A., Menter A. et al. Efficacy, safety, and patient-reported outcomes in patients with moderate-to-severe plaque psoriasis treated with brodalumab for 5 years in a long-term, open-label, phase II study. Am J Clin Dermatol 2019; 20 (6): 863–871, doi: 10.1007/s40257-019-00466-2.
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
Labels
Dermatology & STDs Paediatric dermatology & STDsPopular this week
- How Compassion Fatigue Threatens Doctors and Other Healthcare Workers
- A device the size of a matchbox will help treat obstructive sleep apnea
- Good, Bad, or Ugly? What's the Deal with Eggs?
- Non-Invasive Analysis of Gut Microbiome Biomarkers Enhances Diagnosis of IBD
- New Algorithm to Enhance Prediction of Cardiovascular Disease Risk
Recommended for you
- How to Choose the Ideal Venotonic or Vasoprotective – Which Properties Are Key?
- Pain Relief and Functional Score Improvement in Patients with Low Rheumatoid Arthritis Activity: Baricitinib vs. Adalimumab
- The Wound Healing Process Step by Step and What Can Complicate It
- Diosmin – Still an Important Modality in the Treatment of Venous Insufficiency
- Another Positive Effect of Diosmin − Influence on Oxidative Stress
- Efficacy and Speed of Pain Relief in Rheumatoid Arthritis When Adding Baricitinib to Methotrexate

